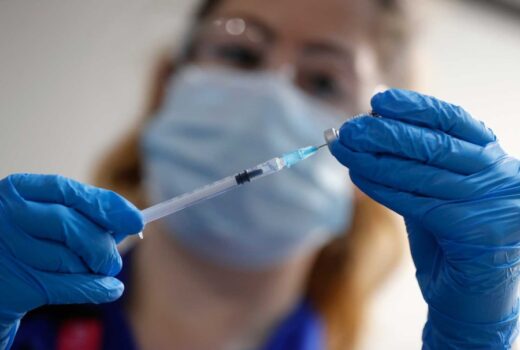
 A U.S. government advisory panel endorsed widespread use of Pfizer’s coronavirus vaccine on Thursday.
A U.S. government advisory panel endorsed widespread use of Pfizer’s coronavirus vaccine on Thursday.
In a 17-4 vote with one abstention, the government advisers concluded that the vaccine from Pfizer and its German partner BioNTech appears safe and effective for emergency use in adults and teenagers 16 and over.
The crucial endorsement came despite questions about allergic reactions in two people who received the vaccine earlier this week in the United Kingdom.
Pfizer has said it will have about 25 million doses of the two-shot vaccine for the U.S. by the end of December. However, the initial supplies will be reserved primarily for health care workers and nursing home residents.
The decision came as COVID-19 cases surge to ever-higher levels across the U.S., with deaths setting an all-time, one-day record of more than 3,100 on Wednesday.
Global Markets Plunge As Trump’s Sweeping Tariffs Spark Recession Fears
Devastating 7.7 Magnitude Earthquake Rocks Southeast Asia, Killing Multiple People
Trump Announces Sweeping 25% Tariffs On Imported Cars And Parts
Sensitive US Military Plans Leaked In Stunning Security Breach