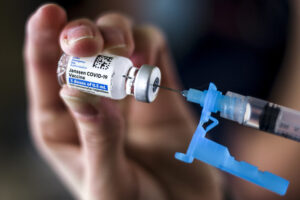
Federal health agencies on Tuesday called for an immediate pause in use of Johnson & Johnson’s single-dose coronavirus vaccine after six recipients in the United States developed a rare disorder involving blood clots within about two weeks of vaccination.
All six recipients were women between the ages of 18 and 48. One woman died and a second woman in Nebraska has been hospitalized in critical condition.
The Food and Drug Administration (FDA) said it was acting “out of an abundance of caution”.
The recommendation follows similar rare cases in the AstraZeneca vaccine, which has prompted some curbs in its use.
Nearly seven million people in the United States have received Johnson & Johnson shots so far, and roughly nine million more doses have been shipped out to the states, according to data from the Centers for Disease Control and Prevention.
“We are recommending a pause in the use of this vaccine out of an abundance of caution,” Dr. Peter Marks, director of the Food and Drug Administration’s Center for Biologics Evaluation and Research, and Dr. Anne Schuchat, principal deputy director of the C.D.C., said in a joint statement. “Right now, these adverse events appear to be extremely rare.”
Johnson & Johnson issued a statement saying that safety was its “number one priority” and that it shared “all adverse event reports” with the health authorities.
It added: “We are aware that thromboembolic events including those with thrombocytopenia have been reported with Covid-19 vaccines. At present, no clear causal relationship has been established between these rare events and the Janssen (J&J) Covid-19 vaccine.”
White House Says Military Option Open On Greenland Acquisition
PHOTO SPLASH: 2025 PSR NATIONAL AWARDS (THE NIGERIAN ACHIEVEMENT AWARDS AND THE WOMAN OF MERIT GOLD AWARDS BY PEOPLE & POWER)
Trump Calls Case Against Maduro “Infallible” Ahead Of U.S. Court Appearance
South Africa Raids U.S. Refugee Processing Centre, Arrests Seven Kenyans