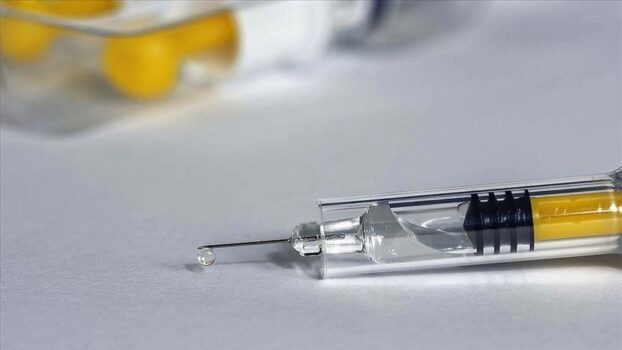
 AstraZeneca (AZN.L) announced on Tuesday its decision to initiate the worldwide withdrawal of its COVID-19 vaccine, citing a surplus of available updated vaccines amid the ongoing pandemic.
AstraZeneca (AZN.L) announced on Tuesday its decision to initiate the worldwide withdrawal of its COVID-19 vaccine, citing a surplus of available updated vaccines amid the ongoing pandemic.
The pharmaceutical giant also revealed its intention to withdraw the marketing authorizations for its vaccine Vaxzevria within Europe.
“As multiple variant COVID-19 vaccines have since been developed, there is a surplus of available updated vaccines,” the company stated. This surplus, AstraZeneca explained, has led to a decline in demand for Vaxzevria, which is no longer being manufactured or supplied.
This decision comes amidst previous admissions by the Anglo-Swedish drugmaker regarding side effects associated with the vaccine, including blood clots and low blood platelet counts, as reported by various media outlets.
According to reports, the firm’s application to withdraw the vaccine was submitted on March 5 and came into effect on May 7. The Telegraph was the first to report this development.
AstraZeneca, listed on the London Stock Exchange, diversified its focus last year, venturing into respiratory syncytial virus vaccines and obesity drugs through several strategic deals. This shift followed a slowdown in growth as sales of COVID-19 medicines declined.
Trump’s Massive Tariffs Take Effect, Deepening Global Trade War
Global Markets Plunge As Trump’s Sweeping Tariffs Spark Recession Fears
Devastating 7.7 Magnitude Earthquake Rocks Southeast Asia, Killing Multiple People
Trump Announces Sweeping 25% Tariffs On Imported Cars And Parts