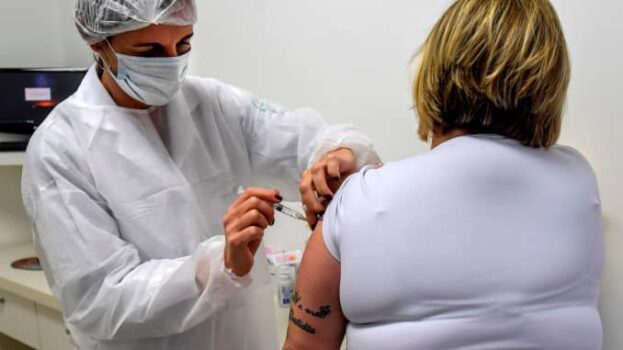
 Pharmaceutical giant, AstraZeneca said Monday that late-stage trials showed its coronavirus vaccine was up to 90% effective against COVID-19. This has raised hopes that the company will soon roll out a vaccine that is easier to distribute than some of its rivals.
Pharmaceutical giant, AstraZeneca said Monday that late-stage trials showed its coronavirus vaccine was up to 90% effective against COVID-19. This has raised hopes that the company will soon roll out a vaccine that is easier to distribute than some of its rivals.
The results are based on interim analysis of trials in the U.K. and Brazil of a vaccine developed by Oxford University and manufactured by AstraZeneca. No hospitalizations or severe cases of COVID-19 were reported in those receiving the vaccine.
“These findings show that we have an effective vaccine that will save many lives,” Oxford University Professor Andrew Pollard, chief investigator for the trial, said in a statement. “Excitingly, we’ve found that one of our dosing regimens may be around 90% effective.”
AstraZeneca is the third major drug company to report late-stage results for its potential COVID-19 vaccine. Pfizer and Moderna last week reported preliminary results from late-stage trials showing their vaccines were almost 95% effective.
However, unlike the Pfizer and Moderna vaccines, the Oxford-AstraZeneca candidate does not have to be stored at ultra-cold temperatures, making it easier to distribute, especially in developing countries. All three vaccines must be approved by regulators before they can be widely distributed.
“The Oxford vaccine can be stored in the fridge, as opposed to the freezer like the other two vaccines, which means it is a more practical solution for use worldwide,” said Peter Horby, professor of Emerging Infectious Diseases and Global Health at Oxford.
The results come as a second wave of COVID-19 hits many countries, once again shutting businesses, restricting social interaction and pummeling the world economy.
ICC Issues Arrest Warrants For Netanyahu, Gallant And Al-Masri
Russia Fires Intercontinental Missile In Ukraine Attack — Kyiv
South African Police Raid Warehouses Amid Food Poisoning Deaths
Russian Missile Strike Kills Eight, Wounds 39 In Ukraine’s Odesa Region